Atlas peripheral stent
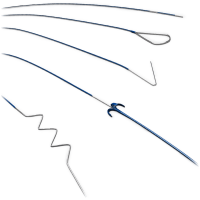
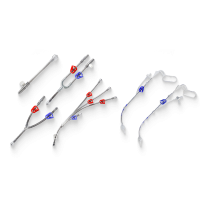
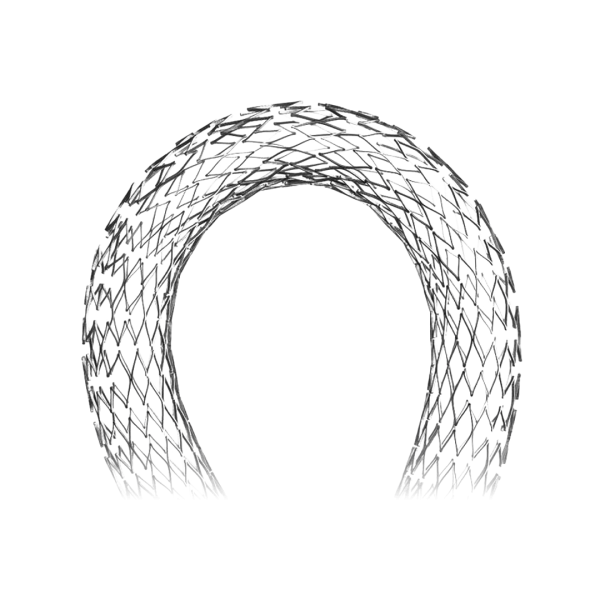
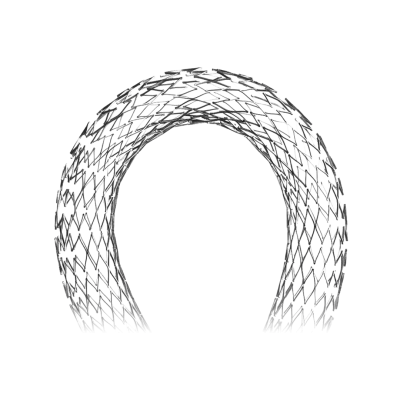
Atlas is a self-expanding peripheral stent designed for lesions in the femoral, iliac, or popliteal arteries, offering reliable radial support and low rates of restenosis in interventions for peripheral arterial disease (PAD).
Atlas peripheral stent
The Atlas peripheral stent sets a new standard in the treatment of peripheral arterial disease, thanks to its robust, flexible, and highly precise design.
Indicated for vessel diameters between 5 mm and 8 mm and lesion lengths from 20 mm to 200 mm, it is ideal for areas at high risk of sudden closure or restenosis following balloon angioplasty (PTA).
Manufactured from nitinol alloy using laser cutting, this self-expanding stent offers a safe and minimally invasive alternative to traditional open surgery.
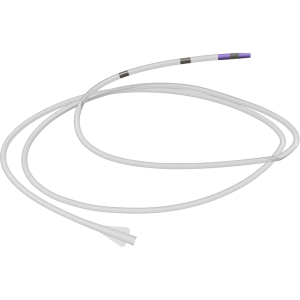
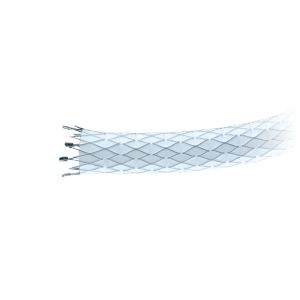
Its triaxial delivery system is designed for stable, controlled, and precise deployment, compatible with 0.035” guidewires and available in catheter lengths of 80 and 120 cm.
Features
- Self-expanding: made from nitinol alloy with shape memory.
- Compatible vessel diameters: from 5 mm to 8 mm.
- Treatable lengths: from 20 mm to 200 mm.
- Triaxial delivery system design for enhanced stability and control.
- 6F outer profile: maximum compatibility with peripheral access sites.
- Compatible with 0.035” (0.89 mm) guidewires.
- Radiopaque markers for precise placement under fluoroscopy.
- Catheter lengths: available in 80 cm and 120 cm.
Benefits
- Improved blood flow in areas affected by PAD.
- Lower risk of restenosis compared to conventional techniques.
- Less invasive procedure than traditional open surgery.
- High deployment precision thanks to its controlled delivery system.
- Versatility to treat various peripheral vascular configurations.
More details
Atlas peripheral stent
Política de privacidad
The Owner informs you about its Privacy Policy regarding the processing and protection of personal data of users that may be collected during browsing through the Website: https://biogendiagnostica.com
In this regard, the Owner guarantees compliance with the current regulations on personal data protection, as reflected in Organic Law 3/2018, of 5 December, on the Protection of Personal Data and Guarantee of Digital Rights (LOPD GDD). It also complies with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons (GDPR).
Use of the Website implies acceptance of this Privacy Policy así como las condiciones incluidas en el Aviso Legal.
Identity of the responsible
- Responsible: BIOGEN DIAGNÓSTICA SL.
- NIF: B81382210
Inscrita en el registro mercantil de Madrid, tomo 10.442, libro 0, sección 157, folio 8, hoja M165916, inscripción 1ª. - Address: CALLE FLORIDA, 1, NAVE 17, 28670 VILLAVICIOSA DE ODÓN, Madrid - España.
- Email address: biogen@biogen-diagnostica.com
- Contact phone number: +34916164054
- Website: https://biogendiagnostica.com
Principles applied in data processing
In the processing of your personal data, the Owner will apply the following principles, which comply with the requirements of the new European data protection regulation (GDPR):
- Principle of lawfulness, fairness, and transparency: The Owner will always require consent for the processing of personal data, which may be for one or several specific purposes about which the Owner will inform the User beforehand with complete transparency.
- Principle of data minimisation: The Owner will request only the data strictly necessary for the purpose or purposes for which they are requested.
- Principle of storage limitation: The Owner will retain the collected personal data only for the time strictly necessary for the purpose or purposes of the processing. The Owner will inform the User of the corresponding retention period according to the purpose.
In the case of subscriptions, the Owner will periodically review the lists and remove records that have been inactive for a considerable period. - Principle of integrity and confidentiality: The personal data collected will be processed in such a way that their security, confidentiality, and integrity are guaranteed.
The Owner takes the necessary precautions to prevent unauthorized access or misuse of its users’ data by third parties.
Collection of personal data
It is not necessary to provide any personal data in order to browse the Website.
Rights
The Owner informs you that regarding your personal data, you have the right to:
- Request access to the stored data.
- Request rectification or deletion.
- Request the restriction of their processing.
- Object to the processing.
No podrá ejercitar el derecho a la portabilidad de los datos.
The exercise of these rights is personal and therefore must be carried out directly by the data subject, by submitting the request directly to the Controller. This means that any client, subscriber, or collaborator who has provided their data at any time may contact the Controller to request information about the data stored and how it was obtained, request its rectification, object to the processing, limit its use, or request the deletion of such data from the Controller’s files.
To exercise your rights, you must send your request along with a photocopy of your National Identity Document or its equivalent to the email address: biogen@biogen-diagnostica.com
The exercise of these rights does not include any data that the Owner is obliged to retain for administrative, legal, or security purposes.
You have the right to effective judicial protection and to file a complaint with the supervisory authority, in this case, the Spanish Data Protection Agency, if you consider that the processing of your personal data violates the Regulation.
Purpose of personal data processing
When you connect to the Website to send an email to the Owner, subscribe to the newsletter , you are providing personal data for which the Owner is responsible. This information may include personal data such as your IP address, full name, physical address, email address, phone number, and other information. By providing this information, you consent to its collection, use, management, and storage by the — Arsys Internet SLU — only as described on the pages:
Personal data and the purpose of processing by the Controller vary depending on the information collection system:
There are other purposes for which the Controller processes personal data:
- Para garantizar el cumplimiento de las condiciones recogidas en la página de Aviso Legal y de la ley aplicable. Esto puede incluir el desarrollo de herramientas y algoritmos que ayuden al Sitio Web a garantizar la confidencialidad de los datos personales que recoge.
- To support and improve the services offered by this Website.
- To analyze user browsing. The Owner collects other non-identifying data obtained through the use of cookies that are downloaded onto the User’s computer when browsing the Website, whose characteristics and purpose are detailed on the page of Política de Cookies.
Security of personal data
To protect your personal data, the Owner takes all reasonable precautions and follows industry best practices to prevent its loss, misuse, unauthorised access, disclosure, alteration, or destruction.
The Website is hosted on: Arsys Internet SLU. Data security is guaranteed, as all necessary security measures are taken to ensure it. You can consult their privacy policy for more information.
The Owner informs the User that their personal data will not be transferred to third-party organisations, except when such data transfer is covered by a legal obligation or when the provision of a service requires a contractual relationship with a data processor. In the latter case, data will only be transferred to the third party when the Owner has the User’s explicit consent.
However, in some cases, collaborations may be carried out with other professionals. In such cases, the User’s consent will be requested, providing information about the identity of the collaborator and the purpose of the collaboration. These will always be conducted with the strictest security standards.
Content from other websites
The pages of this Website may include embedded content (e.g., videos, images, articles, etc.). Embedded content from other websites behaves in exactly the same way as if you had visited the other website.
These websites may collect data about you, use cookies, embed additional third-party tracking code, and monitor your interaction using that code.
Cookies Policy
For this website to function properly, it needs to use cookies, which are pieces of information stored in your web browser.
You can find all the information related to the cookie collection and processing policy on the page of Política de Cookies.
Legal basis for data processing
The legal basis for the processing of your data is:
- The consent of the data subject.
Categories of personal data
The categories of personal data processed by the Data Controller are:
- Identifying data.
- No categories of specially protected data are processed.
Retention of personal data
The personal data provided to the Data Controller will be retained until the data subject requests its deletion.
Recipients of personal data
- Google Analytics is a web analytics service provided by Google, Inc., a Delaware company whose principal office is located at 1600 Amphitheatre Parkway, Mountain View, California, CA 94043, United States (“Google”).
Google Analytics uses “cookies”, which are text files placed on your computer, to help the Owner analyse how Users use the Website. The information generated by the cookie about your use of the Website (including your IP address) will be directly transmitted to and stored by Google on servers in the United States.
More information in: https://analytics.google.com
You can find out how Google manages privacy regarding the use of cookies and other information on Google’s Privacy Policy page: https://policies.google.com/privacy?hl=es
You can also view a list of the types of cookies used by Google and its partners, as well as all the information related to their use of advertising cookies, in:
Navegación Web
While browsing the Website, non-identifiable data may be collected, which may include your IP address, geolocation, a record of how services and sites are used, browsing habits, and other data that cannot be used to identify you.
The Website uses the following third-party analytics services:
- Google Analytics.
The Owner uses the information obtained to gather statistical data, analyse trends, manage the site, study browsing patterns, and collect demographic information.
The Owner is not responsible for the processing of personal data carried out by websites that may be accessed through the various links contained on the Website.
Accuracy and truthfulness of personal data
You agree that the data provided to the Owner is correct, complete, accurate, and up to date, as well as to keep it properly updated.
As a User of the Website, you are solely responsible for the truthfulness and accuracy of the data submitted to the Website, releasing the Owner from any liability in this regard.
Acceptance and consent
As a User of the Website, you declare that you have been informed of the conditions regarding the protection of personal data, and you accept and consent to the processing of such data by the Owner in the manner and for the purposes indicated in this Privacy Policy.
Para contactar con el Titular, suscribirse a un boletín o realizar comentarios en este sitio Web tiene que aceptar la presente Política de Privacidad.
Changes to the Privacy Policy
The Owner reserves the right to modify this Privacy Policy to adapt it to legislative or jurisprudential changes, as well as industry practices.
These policies will remain in effect until they are modified by others duly published.